查看更多
密码过期或已经不安全,请修改密码
修改密码
壹生身份认证协议书
同意
拒绝

同意
拒绝

同意
不同意并跳过





【神经科临床必备】17天轻松干预、处置神经心理问题:

Stroke & Vascular Neurology(SVN)最新上线文章“Preclinical evaluation of ZL006-05, a new antistroke drug with fast-onset antidepressant and anxiolytic effects”,来自南京医科大学药学院朱东亚教授团队等。
卒中后抑郁和焦虑是功能结局不良的独立预测因子,在卒中急性期很常见。迄今为止,尚无适用于急性卒中治疗的快速起效的抗抑郁和抗焦虑药物。研究团队研发的由ZL006衍生的ZL006-05,是一种双靶点镇痛药物,可阻断一氧化氮合酶与突触后致密区蛋白-95的耦联,同时选择性激活含α2的离子型γ-氨基丁酸A型受体(GABAAR)。本研究旨在明确ZL006-05是否可用作具有快速起效抗抑郁和抗焦虑作用的卒中治疗药物。
作者团队在大鼠和小鼠中诱导光血栓性卒中和短暂性大脑中动脉闭塞。采用TTC(2,3,5-Triphenyltetrazolium chloride, TTC)染色或尼氏染色测定梗死面积。采用4分制神经功能评分或改良神经损伤严重程度评分(modified Neurological Severity Scores, mNSS)对神经系统损伤进行评估。通过网格爬行实验、圆筒实验和改良胶带移除实验评估感觉运动功能。采用Morris水迷宫实验(Morris water maze, MWM)评估空间学习能力。利用慢性轻度不可预见性应激刺激模型(unpredictable chronic mild stress, UCMS)诱发抑郁和焦虑。通过悬尾实验、强迫游泳实验和糖水偏好实验(sucrose preference tests, SPTs)评估抑郁行为。通过新奇抑制摄食实验(novelty-suppressed feeding, NSF)和高架十字迷宫实验(elevated plus maze, EPM)评估焦虑行为。同时在大鼠体内进行药代动力学、毒代动力学和长期毒性研究。
研究结果显示,在卒中急性期给予ZL006-05可减轻短暂性及永久性缺血损伤,并在治疗窗口期为缺血后12小时内,显著改善长期功能损伤。同时可减少纤溶酶原激活导致的出血转化。研究还发现,ZL006-05在正常小鼠和慢性轻度应激模型(chronic mild stress, CMS)小鼠中均产生快速起效的抗抑郁和抗焦虑作用,起效潜伏期为1小时,在卒中小鼠中也具有抗抑郁和抗焦虑作用。研究同时证实了ZL006-05可穿过血脑屏障并迅速进入大脑,在毒动力学和长期毒理学研究中具有较高的安全性。
Figure 1. Protective effects of ZL006-05 after cerebral ischaemia/reperfusion. (A) (Upper) The hypothesis: in the acute phase of stroke, NO overproduction caused by increased nNOS-PSD-95 interaction, and GABAARs dysfunction lead to ischaemic damage and depression and/or anxiety. ZL006-05 prevents the ischaemic damage and produces fast-onset antidepressant and/or anxiolytic effects by (1) reducing NO production via uncoupling nNOS-PSD-95 and (2) potentiating α2-containing GABAAR. (Lower) chemical structure of ZL006-05. (B, C) Effects of ZL006-05 in male rats. n=8 for sham, n=15–16 for other groups. (B) Representative cerebral infarct images stained using TTC in coronal sections of rat brains (upper) and infarct volume presented as a percentage of the intact hemisphere (lower) (one-way ANOVA, F6,94=13.494. ***p<0.001, vs sham; ###p<0.001, #p=0.024, vs stroke/vehicle). (C) Neurological scores (Mann-Whitney U test, ***p<0.001, vs sham; ###p<0.01, vs vehicle/stroke). (D, E) Effects of ZL006-05 in female rats. n=8 for sham, n=17–18 for other groups. (D) Representative cerebral infarct images in coronal sections of rat brains (upper) and infarct volume presented as a percentage of the intact hemisphere (lower) (one-way ANOVA, F5,89=16.580. ***p<0.001, vs sham; ###p<0.001, ##p=0.006, vs stroke/vehicle). (E) Neurological scores (Mann-Whitney U test, ***p<0.001, vs sham; ###p<0.01, #p=0.011, vs vehicle/stroke). ANOVA, analysis of variance; ED, edaravone dexborneol; GABAAR, γ-Aminobutyric acid type A receptor; NMDAR, N-methyl-D-aspartate receptors; nNOS, nitric oxide synthase; TTC, 2,3,5-Triphenyltetrazolium chloride.
Figure 2. Therapeutic time window of ZL006-05 after permanent focal cerebral ischaemia. (A–E) Effects of ZL006-05 in the mice subjected to photothrombotic stroke. n=14. (A) Foot faults of the left forelimb (one-way ANOVA, F3,52=89.690. ***P < 0.001, vs sham; ###p<0.01, ###p<0.001, vs vehicle/stroke) and (B) foot faults of the right forelimb (one-way ANOVA, F3,52=0.031, p=0.993) in the grid-walking task. (C) Forelimb symmetry in the cylinder task (one-way ANOVA, F3,52=149.963. ***p<0.001, vs sham; ###p<0.001, vs vehicle/stroke). (D) Representative cerebral infarct images stained using Nissl and (E) Bar graph showing infarct size at 11 d after stroke (one-way ANOVA, F3,52=59.533. ***p<0.001, vs sham; ##p<0.01, vs vehicle/stroke). (F–J) Effects of ZL006-05 in the rats subjected to photothrombotic stroke. n=13. (F) Foot faults of the left forelimb (one-way ANOVA, F3,48=291.84. ***p<0.001, vs sham; ###p<0.001, #p=0.028, vs vehicle/stroke) and (G) foot faults of the right forelimb (one-way ANOVA, F3,48=0.034, p=0.991) in the grid-walking task. (H) The ratio of left to right performance on the modified sticky-tape test (one-way ANOVA, F3,48=320.54. ***p<0.001, vs sham; ##p=0.003, #p=0.049, vs vehicle/stroke). (I) Representative cerebral infarct images stained using Nissl and (J) bar graph showing infarct size on d seven after stroke (one-way ANOVA, F3,48=67.34. ***p<0.001, vs sham; ##p=0.001, #p=0.010, vs vehicle/stroke). ANOVA, analysis of variance; ED, edaravone dexborneol.

Figure 3. Long-term effects of ZL006-05 after stroke. (A–E) Effects of ZL006-05 in the rats subjected to photothrombotic stroke, in which, 3d and 7d are consecutive days of drugs administration after stroke. n=14. (A) Experimental design for B–E) (B) Foot faults of the left forelimb (one-way ANOVA, F4,65=115.161. ***P<0.001, vs sham; ###p<0.001, vs vehicle/stroke) and (C) foot faults of the right forelimb (one-way ANOVA, F4,65=0.008, p=1.000) in the grid-walking task. (D) The ratio of left to right performance on the modified sticky-tape test (one-way ANOVA, F4,65=216.323. ***p < 0.001, vs sham; ##p<0.01, ###p<0.001, vs vehicle/stroke). (E) Representative cerebral infarct images stained using Nissl (left) and bar graph showing infarct size at 31 d after stroke (right) (one-way ANOVA, F4,65=38.474. ***p<0.001, vs sham; ###p<0.001, vs vehicle/stroke). (F–J) Effects of ZL006-05 in the rats subjected to MCAO/reperfusion, in which, 3d and 7d are consecutive days of drugs administration after cerebral ischaemia. n=13–14 for sham, n=16–18 for other groups. (F) Experimental design for (G–J) (G) Modified neurological severity score (Mann-Whitney U test, ***p<0.001, vs sham; for ##p<0.01, #p=0.030, vs vehicle/stroke). (H) Foot faults of the left forelimb (one-way ANOVA, F4,79=101.549. ***p<0.001, vs sham; ###p<0.001, vs vehicle/stroke) and (I) foot faults of the right forelimb (one-way ANOVA, F4,79=0.111, p=0.978) in the grid-walking task. (J) Escape latency in Morris water maze task (two-way repeated-measures ANOVA, F7,315=29.620. ***p<0.001, Vehicle/sham vs vehicle/stroke; ##p=0.0015, ED/stroke vs vehicle/stroke; ##p=0.007, ZL006-5 (3d)/stroke vs vehicle/stroke; ##p=0.0058, ZL006-05 (7d)/stroke vs vehicle/stroke). (K) Swimming speed in Morris water maze task (F4,79=0.259, p=0.903). ANOVA, analysis of variance; ED, edaravone dexborneol; MCAO, middle cerebral artery occlusion.
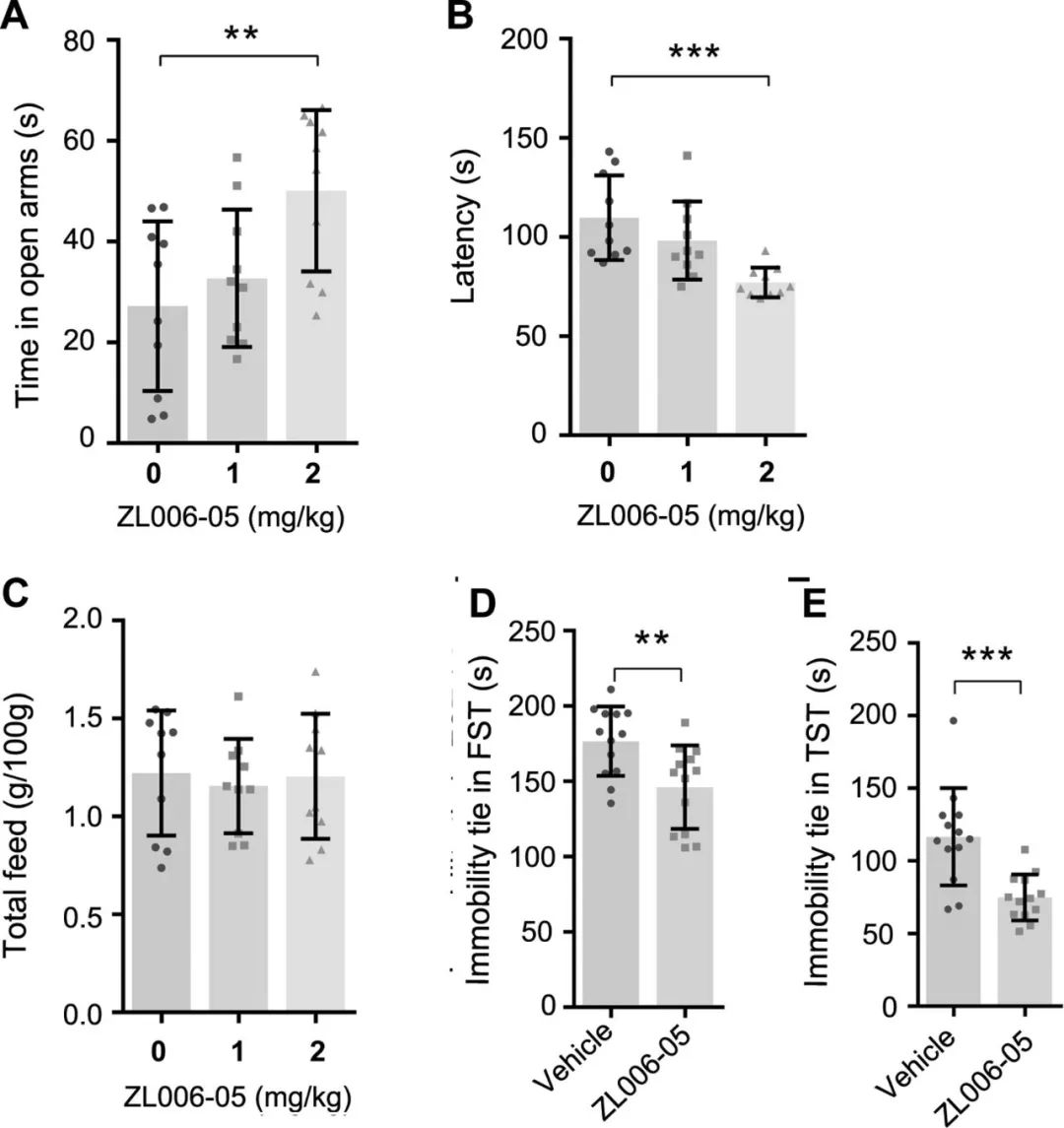
Figure 4. ZL006-05 produces fast-onset anxiolytic-like and antidepressant-like effects. (A–E) Effects of ZL006-05 in mice. (A–C), n=10; (D, E), n=13. (A) Time spent in open arms in EPM test (one-way ANOVA, F2'27279.206, **P = 0.003), (B) Latency in the NSF test (one-way ANOVA, F2'27279.206, **P < 0.001). (C) Food consumption in the NSF test (one-way ANOVA, F2'27270.134, p=0.875). (D) Immobility time in the FST test (**p=0.005, two-tailed t-test). (E) Immobility time in the TST test (***p<0.001, two-tailed t-test). ANOVA, analysis of variance; EPM, elevated plus maze; NSF, novelty-suppressed feeding; TST, tail suspension test.
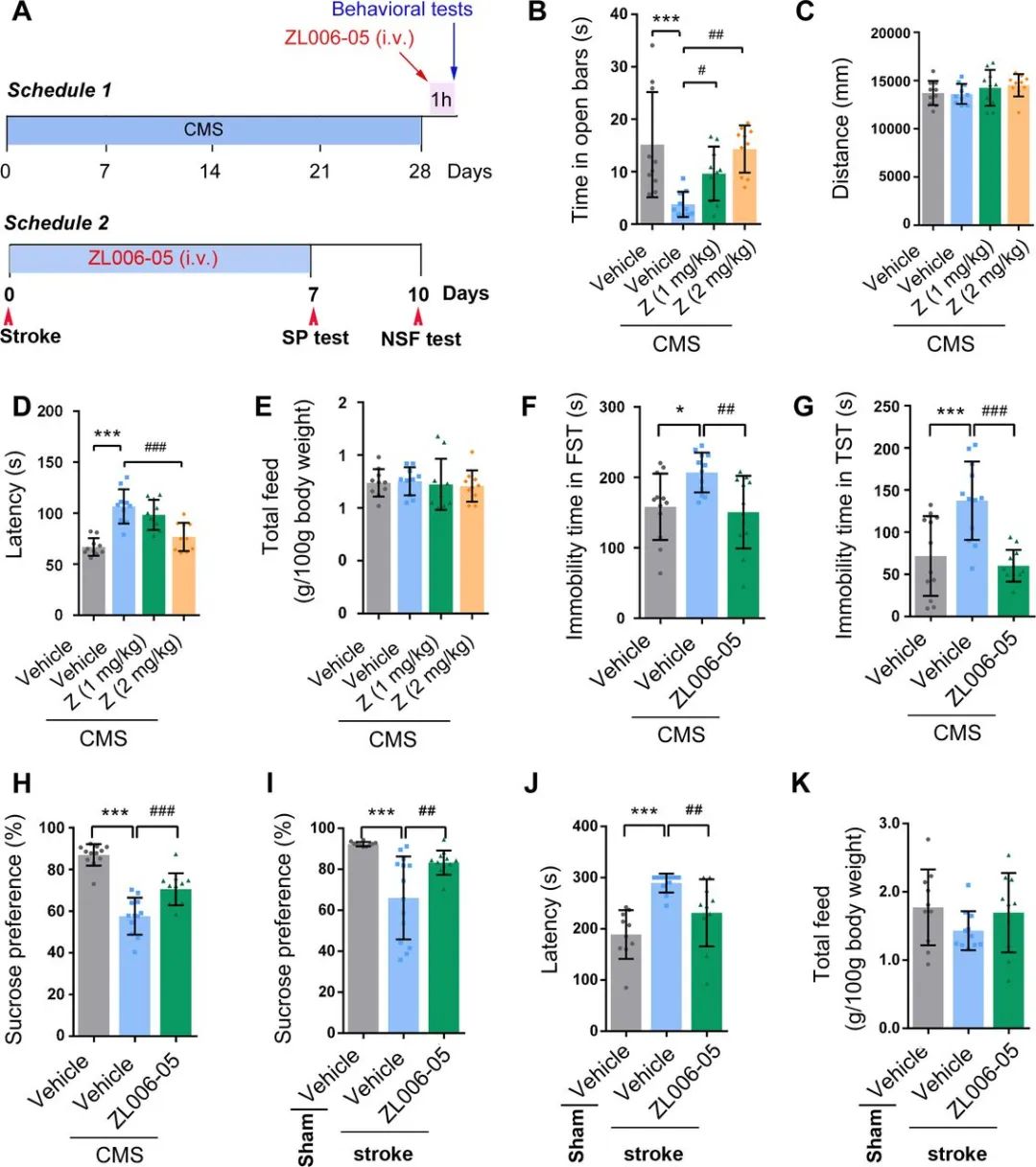
Figure 5. ZL006-05 reverses UCMS-induced and stroke-induced anxiety and depression in a fast acting manner. (A) Experimental designs for (B–H) (upper) and (I–K) (lower). (B–E), n=10; (F–H), n=12. (B) Time spent in open arms in EPM test (one-way ANOVA, F3'36367.122, ***p<0.001, #p=0.042, ##p=0.001). (C) Locomotor activities in the open field test (one-way ANOVA, F3'36360.997, p>0.05, between groups). (D) Latency in the NSF test (one-way ANOVA, F3'363617.782, ***p<0.001, ####p<0.001). (E) Food consumption in the NSF test (one-way ANOVA, F3'36360.133, p>0.05, between groups). (F) Immobility time in the FST test (one-way ANOVA, F2,33=5.92, *p=0.01, ##p=0.003). (G) Immobility time in the TST test (one-way ANOVA, F2,33=13.173, ***p<0.001, ###p<0.001). (H) Sucrose preference in the SPT test (one-way ANOVA, F2,33=47.186, ***p<0.001, ###p<0.001). (I) Sucrose preference in the SPT test (one-way ANOVA, F2,33=12.481. ***p<0.001, ##p=0.020; n=10, for sham; n=14, for tMCAO+vehicle; n=12, for tMCAO+ZL006-05). (J) Latency in the NSF test (one-way ANOVA, F2'282811.982. ***p<0.001, ##p=0.009; n=10, for sham and tMCAO+ZL006-05; n=11, for tMCAO+vehicle). (K) Food consumption in the NSF test (one-way ANOVA, F2'28281.436, p>0.05, between groups, n=10, for sham and tMCAO+ZL006-05; n=11, for tMCAO+vehicle). ANOVA, analysis of variance; EPM, elevated plus maze; NSF, novelty-suppressed feeding; SPT, sucrose preference test; tMCAO, transient middle cerebral artery occlusion; TST, tail suspension test; UCMS, unpredictable chronic mild stress.
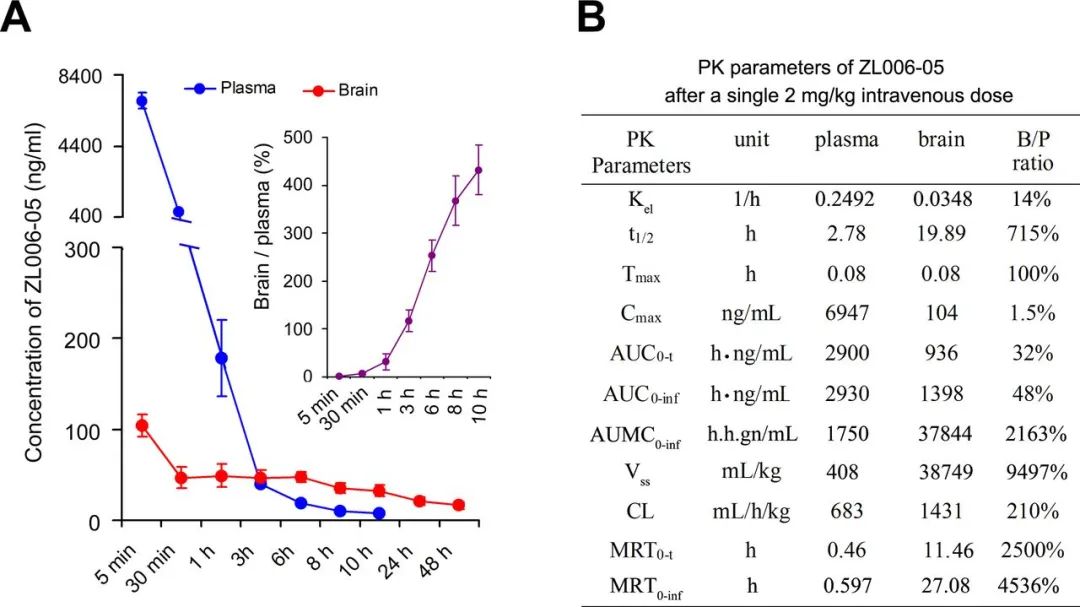
Figure 6. Time-concentration profiles of ZL006-05 in plasma and brain tissue and PK parameters after a single 2 mg/kg intravenous dose in male rats. (A) Concentrations of ZL006-05 in plasma and brain tissue and the ratio of brain/plasma. n=3, each time point. (B) Mean pharmacokinetics parameters of ZL006-05 in brain tissue and plasma. ZL006-05 was intravenously injected immediately after reperfusion. AUC, area under curve; B, brain; P, plasma; PK, pharmacokinetics.
研究结论,ZL006-05是一种具有快速起效抗抑郁和抗焦虑作用的新型神经保护剂,在疗效、安全性和临床问题靶向方面具有转化特性。

来源:SVN俱乐部
转载已获授权,其他账号转载请联系原账号
国家神经系统疾病质控中心脑血管病专业组专家撰稿,21篇脑梗死临床诊疗干货文章,精准提升脑血管临床诊疗能力!
四川大学华西医院周东教授牵头,国家神经系统疾病质控中心癫痫专业组专家撰稿18篇精品干货文章,手把手指导癫痫及癫痫持续状态临床诊疗。
复旦大学附属华山医院董强教授牵头,程忻教授主编,分享卒中领域最新指南及进展,以前沿视角审视卒中诊疗!
神经影像解剖及血供范围分布图-颅脑CT【轴位】
神经影像解剖及血供范围分布图-颅脑CT/MR【矢状位】
ESVS 2023最新临床实践指南解读:颈/椎动脉粥样硬化性疾病指南新增建议【华山卒中】
详细的颅脑MRI正常解剖图谱,收藏级!【T1WI冠状位解剖图】
详细的颅脑MRI正常解剖图谱,收藏级!【T2WI反转序列冠状位解剖图】
详细的颅脑MRI正常解剖图谱,收藏级!【T2WI反转序列轴位解剖图】
详细的颅脑MRI正常解剖图谱,收藏级!【T2WI矢状位解剖图】
详细的颅脑MRI正常解剖图谱,收藏级!【T2WI轴位解剖图】
详细的颅脑MRI正常解剖图谱,收藏级!【T2WI冠状位解剖图】
详细的颅脑MRI正常解剖图谱,收藏级!【T 1 WI轴位解剖图】
详细的颅脑MRI正常解剖图谱,收藏级!【T1WI矢状位解剖图】
查看更多